Stirred Tank Bioreactors, Mini Bioreactors, Fermenters, and Multistage Bioreactors: Advances and Applications in Biotechnology
In the realm of biotechnology, the development and utilization of various types of bioreactors have played a crucial role in driving innovation and progress. This article explores the features, workings, and applications of stirred tank bioreactors, mini bioreactors, fermenters, and multistage bioreactors, highlighting their significance in modern biotechnological processes.
Stirred tank bioreactors are among the most commonly used systems in industrial and research settings. These bioreactors consist of a cylindrical vessel with a mechanical agitator to ensure uniform mixing of the contents. The agitation helps in distributing nutrients, oxygen, and heat evenly throughout the reactor, creating a homogeneous environment for cell growth and metabolite production. The design of the agitator is critical, as it should provide sufficient mixing without causing excessive shear stress that could damage the cells.
The controlled parameters in stirred tank bioreactors include temperature, pH, dissolved oxygen levels, and substrate concentration. Sophisticated monitoring and control systems are employed to maintain these parameters within the optimal range for the specific biological process. This precise control is essential for achieving high productivity and consistent product quality.
Mini bioreactors have gained popularity in recent years, especially in early-stage research and process development. Their small size offers several advantages, such as reduced reagent consumption, faster experimentation, and easier handling. Despite their smaller volume, mini bioreactors are designed to replicate the key features of larger-scale systems, allowing for reliable data generation and scalability assessment.
Mini bioreactors are often equipped with miniaturized sensors and control systems that provide real-time data on critical parameters. This enables researchers to quickly optimize culture conditions and evaluate different strategies before moving on to larger-scale production. Their portability and ease of use also make them suitable for high-throughput screening applications, where multiple conditions can be tested simultaneously.
Fermenters are specialized bioreactors commonly used in the production of microbial metabolites, such as antibiotics, enzymes, and biofuels. They are designed to support the growth and metabolic activities of microorganisms under controlled conditions. The choice of fermenter type depends on the specific requirements of the microbial strain and the nature of the product being synthesized.

For example, aerobic fermenters are equipped with efficient aeration systems to supply sufficient oxygen to the microorganisms. Anaerobic fermenters, on the other hand, are designed to maintain an oxygen-free environment for the growth of anaerobic organisms. The design of the fermenter also takes into account factors such as pH control, foam management, and the removal of metabolic by-products to ensure the efficient and stable operation of the fermentation process.
Multistage bioreactors offer a more complex and flexible approach to bioprocessing. These systems consist of multiple compartments or stages, each with its own set of controlled parameters. This allows for the sequential or simultaneous execution of different reactions or cell growth phases within a single reactor.
Multistage bioreactors can be used to simulate complex natural processes or to optimize the production of multiple products from a single bioprocess. For instance, in a multistage bioreactor for wastewater treatment, different stages can be dedicated to the removal of specific contaminants or the growth of different microbial communities for enhanced treatment efficiency.
The applications of these bioreactor types are vast and diverse. In the pharmaceutical industry, stirred tank bioreactors are used for the production of therapeutic proteins and monoclonal antibodies. Mini bioreactors help in the early discovery and optimization of cell lines and culture conditions. Fermenters play a crucial role in the large-scale production of antibiotics and vaccines.
In the field of biofuels, fermenters and multistage bioreactors are employed to convert biomass into ethanol or other bio-based fuels. The food industry benefits from these technologies in the production of enzymes, flavors, and food additives. Environmental biotechnology uses bioreactors for wastewater treatment and the degradation of pollutants.
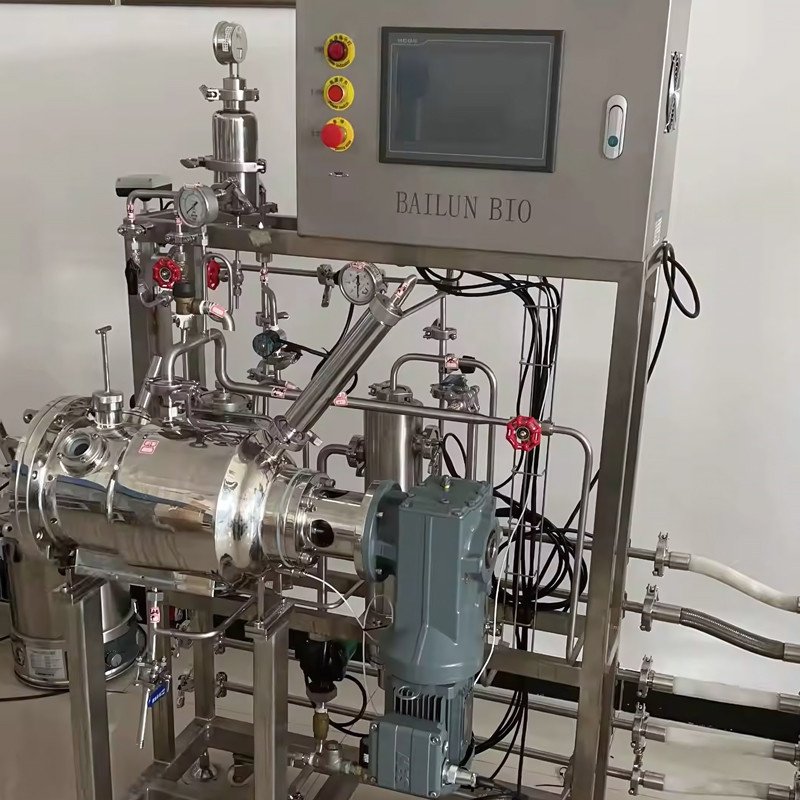
However, the operation and maintenance of these bioreactors come with their challenges. Maintaining sterility to prevent contamination is of utmost importance, especially in large-scale operations. The calibration and reliability of sensors and control systems are crucial for accurate process monitoring and control. Scaling up from laboratory-scale to industrial-scale bioreactors requires careful consideration of engineering factors to ensure consistent performance.
Advancements in materials science, sensor technology, and process control are constantly improving the performance and capabilities of these bioreactors. The integration of artificial intelligence and machine learning algorithms is enabling predictive maintenance and optimization of bioprocesses. New designs and configurations are emerging to address specific challenges and meet the evolving demands of the biotechnology sector.
In conclusion, stirred tank bioreactors, mini bioreactors, fermenters, and multistage bioreactors are essential tools in biotechnology. Their continuous development and application are driving the advancement of various industries, from healthcare and energy to food and environmental protection. The future holds even greater potential as we continue to innovate and optimize these bioreactor systems for more efficient and sustainable bioprocesses.


